Maggi
Administrator
The topic is currently being worked on. As of 18.12.2025
Reliable pKI and nM values are still unclear

Sources: NIMH PDSP - UNC APExBIO fishersci.de Psych Scene Hub benchchem.com MDPI.
Note on the evidence: Ratings in the table are based on your specification combined with typical clinical experiences; The strength of evidence varies greatly between combinations (some are well studied, others only in case series). Concrete therapy decisions should always be made in an interdisciplinary and patient-specific manner.
Reliable pKI and nM values are still unclear
Mechanisms of action in therapy-resistant schizophrenia
The question of "what exactly works" in therapy resistance (TRS) can only be answered in a differentiated way: It is rarely a single receptor. In most cases, a viable dopaminergic axis (D2/D3/D4), modulated by serotonin (5-HT), and, depending on the patient, a limited, specifically used "broad profile" for sleep, anxiety, affect and cognition is required. Below are the key mechanisms, meaningful targets (in vivo), benefit/risk of add-ons such as quetiapine, and the specifics of clozapine — including uncertainties.Receptor axes that carry the antipsychotic effect
- D2 (striatum, limbic):
- Role: Core mechanism for reducing positive symptoms.
- Objective in vivo: Acutely typically about 60–80% receptor occupation; >80% increases EPS/prolactin risk, <60% often insufficiently.
- Note: Partial agonists stabilize the axis with less EPS/prolactin.
- D3 (mesolimbic, prefrontal):
- Role: Motivation, reward, negative symptoms; Cariprazine focuses here.
- Risk: Excessive blockage can trigger akathisia/internal pressure; Dose fine-tuning is crucial.
- D4 (prefrontal, limbic):
- Role: Modulation of positive symptoms/cognition in TRS; clozapine-typical component.
- Practically covered by: Clozapine; Partial agonists (aripiprazole, brexpiprazole).
- Uncertainty: Clinical proportion of D4 vs. "broad profile" has not been conclusively clarified — probably synergistically.
- 5-HT2A/5-HT1A (serotonergic):
- 5-HT2A antagonism: lowers EPS risk, modulates glutamate/dopamine, helps with sleep/anxiety.
- 5-HT1A partial agonism: anxiolytic, antidepressant, potentially cognitively favorable; relevant for aripiprazole/brexpiprazole.
- Glutamate/GABA (indirect):
- Role: clozapine affects glutamatergic transmission (possible TRS lever); M1/M4 axes and NMDA modulation are discussed.
- Uncertainty: Target variables in vivo not standardized; clinically relevant, but difficult to quantify.
Target variables: in vivo occupation and practical pKi classification
- D2 Target:
- Acute: ~70% occupancy at D2 is often optimal.
- Condition: as low as possible, but stable; Partial agonists allow fewer side effects with similar clinical stability.
- D3/D4 objectives:
- D3: sufficient, but not maximum — "too much" increases akathisia; with cariprazine, careful titration.
- D4: relevant coverage useful for TRS; can be achieved via clozapine or partial agonists.
- pKi guardrails (as a relation, not absolute):
- D4 high: Clozapine (~8–8.5), aripiprazole (~8.2), brexpiprazole (~8.0).
- D4 moderate/low: Olanzapine (~7.2), risperidone (~7.7), quetiapine (~6.5).
- Rule: Compare the same measurement system; Interpret pKi only in the context of dose/PK/dissociation kinetics.
What makes clozapine effective
- Multiple levers at the same time:
- D4 affinity: provides additional dopaminergic modulation beyond D2.
- Serotonergic/histaminergic/adrenergic: 5-HT2A, H1, α1 contribute to sedation, anxiety reduction and indirect dopaminergic modulation.
- Cholinergic (M1/M4): possible cognitive and glutamatergic effects, but also anticholinergic side effects.
- Net effect: In true TRS, clozapine is often effective because it "adjusts" multiple pathways at once.
- Price: Hematology (agranulocytosis), myocarditis, seizure threshold, severe metabolic load, hypersalivation — require strict monitoring and speak for low doses, add-on use and exit plan.
Is a broad receptor profile absolutely necessary?
- Direct answer: Not necessarily. A viable combination of dopaminergic axis (D2/D3/D4 depending on the profile) + serotoninergic compensation (5-HT2A/5-HT1A) is often sufficient.
- When "broad profile" helps: In cases of pronounced restlessness, sleep disorders, anxiety, affective dysregulation — H1/α1/M1 are helpful here, but they increase sedation, cognitive load and metabolic risks.
- Pragmatic consequence:
- Use the wide profile as a "module" (add-on, low dose), not as a permanent main effect.
- Avoid high load on M1/H1/α1 in maintenance if the dopaminergic axis is stable.
Quetiapine as an add-on without D4 effect: does it have any benefits?
- Yes, as a functional module:
- Use & Benefits: sleep, anxiety, affective stabilization; 5-HT1A partial effects; H1-based sedation.
- Prerequisite: A D2/D3/D4-bearing base (e.g., aripiprazole/brexpiprazole/lurasidone/amisulpride) should provide the main antipsychotic effect.
- Dose: low (typically <150 mg in the evening), limited in acute/transitional phases; subsequent regression to monotherapy.
- Not useful: as monotherapy for TRS or in high doses for "antipsychotic" effects — cognitive/metabolic disadvantages predominate.
Avoiding side effects: Structures instead of intensification
- Dose sparing:
- D2 as close as possible to the lower effective range; avoid duration >80% occupancy.
- Prefer partial agonists when tolerance is central; they relieve prolactin/EPS.
- Add-on as a module, not as a second main therapy:
- quetiapine low for sleep/anxiety;
- Clozapine low only when needed, strictly monitored.
- Monitoring and personalization:
- ECG/QTc for ziprasidone/amisulpride/olanzapine and clozapine combinations;
- Metabolics (weight, glucose, lipids) in H1-strong preparations;
- Prolactin/EPS in D2-strong antagonists;
- Specific vigilance for akathisia in D3-stressed strategies (cariprazine).
Where are the uncertainties?
- D4 contribution to clinical effect: plausible, but not clearly quantified; Head-to-head combination studies are lacking.
- Glutamatergic involvement with clozapine: well-founded hypothesis, but clinical outcomes are inconclusive.
- Gender-specific QTc risks: Signals are inconsistent; local register data is required.
- Long-term consequences of strong D3 blockade: potentially akathisia/Pisa-like patterns; conservative dosage and observation are required.
- pKi comparability: reliable only within consistent measurement systems; in vivo occupancy and PK/PD must also be considered.
Principles that can be implemented directly
- Secure the axis first: a D2-strong and/or partial agonist base (aripiprazole/brexpiprazole; cariprazine with caution in case of negative symptoms) stabilizes the positive symptoms without unnecessary burden.
- Then add modularly: quetiapine low for sleep/anxiety; Clozapine low as a last resort modulus when D4/glutamate levers are needed.
- Back to simplicity: Combinations are temporary, then regress to monotherapy at the lowest effective dose.
- Tolerability before intensification: Clearly separate symptoms and side effects; in the case of "side effect ≈ symptom", it is better to simplify and re-evaluate than to use high doses.
Receptor axes and clinical effects in therapy-resistant schizophrenia
The effect is rarely caused by a single receptor. It is supported by a dopaminergic axis (D2/D3/D4) with serotoninergic compensation (5-HT2A/5-HT1A). A "broad profile" (M1/H1/α1) can modulate symptoms such as sleep/anxiety, but often increases side effect burden. The tables below bundle clinical effects and derive pKi/in vivo targets for practical decisions DocCheck Springer physicianCME.Receptor axes and clinical effects
| Receptor axis | Primary clinical effects | Secondary effects | Typical side effects | Role at TRS |
|---|---|---|---|---|
| D2 (striatum/limbic) | Positive symptoms ↓, antipsychotic core mechanism | EPS/prolactin risk is controlled by the degree of occupancy | EPS, hyperprolactin at high occupancy | Necessary, but >80% staffing often counterproductive |
| D3 (mesolimbic/pFC) | Negative symptoms/motivation, drive | Affective stabilization possible | Akathisia in case of overblockade | Useful, dose tolerance limited (cariprazine) |
| D4 (pFC/limbic) | TRS active component (clozapine, partial agonists) | Cognitive modulation | Tolerability depends on the accompanying profile | Likely synergistic, not sole leverage |
| 5-HT2A (cortical) | EPS ↓, Sleep/Anxiety, Glutamate/DA Modulation | Affective stabilization | Sedation, blood pressure (α1 cross-profile) | Important compensation to the D2 blockade |
| 5-HT1A (somatodendritic) | Anxiolysis, Antidepressant, Cognition | Unrest/internal tension can be dampened | Nausea rare | Contributes to tolerability/function (Ari/Brx) |
| M1/M4 (cholinergic) | Cognitive/glutamate modulation | Intestinal motility | Anticholinergic load (constipation, cognitive loss) | Useful as a module, often harmful in high doses |
| H1 (histamergic) | Sleep, anxiety | Appetite ↑ | Sedation, Weight ↑ | Add-on for acute phases, non-load-bearing for core action |
| α1 (adrenergic) | Calming, Fear ↓ | Orthostasis | Dizziness, hypotension | Helpful in the short term, stressful in the long term |
Key statements for practice
- Load-bearing effect: D2 axis (~60–80% in vivo), supplemented by D3/D4 depending on the profile; 5-HT2A/5-HT1A balance efficacy and tolerability MSD ManualsSpringer.
- Clozapine: acts through D4 + wide, partly glutamate-modulating profile; the load (hematological, metabolic, cognitive) makes it the last resort. A low add-on insert can specifically supplement missing D4 component, but must be strictly monitored in MSD Manuals.
- Quetiapine without D/D4: Yes — as an add-on module for sleep/anxiety/affect if there is a dopaminergic basis. Monotherapy for TRS is not useful; low-dose <150 mg and use DocCheckarztCME.
- Wide profile vs. "axles": A broad profile is not mandatory. Dopamine (D2/D3/D4) + serotonin (5-HT2A/5-HT1A) are often sufficient. H1/M1/α1 are modular levers for acute phases, but are harmful to the maintenance of DocCheckSpringerMSD Manuals.
- Prevention of side effects: dose-friendly close to ED50–ED95 instead of above; Prioritize partial agonists, low-dose add-ons, monitor ECG/metabolic/prolactin; Limit the time of polypharmacy and plan to return to monotherapy SpringerPsychiatrie Verlag.
Uncertainties and open points
- D4 proportion vs. broad profile: the specific clinical contribution of D4 remains methodologically difficult to define; Head-to-head add-on studies are lacking in DocCheckSpringer.
- Long-term consequences of D3 focus: high D3 blockade can trigger akathisia/inner restlessness; Long-term data are limited — conservative dosing makes sense MSD Manuals.
- pKi comparability: only reliable within uniform assays; in-vivo occupancy and PK/dissociation kinetics are mandatory for clinical interpretation by DocCheckphysicianCME.
Transient receptor binding profile table to make it easier to classify profiles
The best available in vitro Ki values (nM) for D2, D3 and D4 compiled; many values vary between studies — I therefore give areas and mark uncertainties. Use these numbers as reference points, not as exact clinical levels NIMH PDSP - UNC APExBIO fishersci.de.Comparison Table (Ki in nM; lower number = higher affinity)
| Active ingredient | D2 (nM) | D3 (nM) | D4 (nM) |
|---|---|---|---|
| Amisulpride | 2.8–4.0 | 3.0–6.0 | >1000 (negligible) |
| Ziprasidon | 0.5–5.0 | 10–50 (variable) | 100–1000 (low) |
| Flupentixol | 1–10 (older data, variable) | 10–100 (unsure) | 50–500 (unsure) |
| Clozapine | 100–200 | 300–700 | 20–40 (relatively high D4 affinity) |
| Quetiapine | 100–300 | 300–1000 | 1000–3000 (very weak) |
| Aripiprazole | 0.3–1.0 | 0.5–1.5 | 30–60 (moderate) |
| Brexpiprazole | 0.5–2.0 | 1–10 | 50–200 (low-moderate) |
| Cariprazine | 0.4–1.0 | 0.05–0.2 (D3 preferred) | >100 (non-primary) |
| Lurasidon | 0.9–2.0 | >50–100 | >1000 (negligible) |
| Risperidone | 2–6 | 10–50 | 50–150 (low) |
| Paliperidone | 2–10 | 10–100 | 50–200 |
| Olanzapine | 8–15 | 50–200 | 10–40 (relatively high D4 affinity) |
Important: Values are taken from different in-vitro radioligand (Ki) assays; Assay conditions, radioligand, species, and laboratory all influence absolute numbers. Areas reflect this heterogeneity and literature uncertainty NIMH PDSP - UNC Psych Scene Hub benchchem.com.
Explanation and practical interpretation
- Clozapine and olanzapine show relatively low Ki values at D4 (i.e. higher affinity) in several profiles — this explains the often discussed D4 component of their pharmacology; Clozapine is most consistently described here benchchem.com NIMH PDSP - UNC.
- Amisulpride is highly selective for D2/D3 (low nM values) and has virtually no clinically relevant D4 binding APExBIO fishersci.de.
- Cariprazine is clearly D3-preferred (extremely low D3-Ki), D4 binding is not dominant; this distinguishes it functionally from classic atypical MDPIs.
- Aripiprazole shows moderate D4 affinity in some profiles, but its clinical effect is primarily determined by D2/D3 partial agonism and 5-HT activity, according to Psych Scene Hub.
- Older typical neuroleptics (e.g., flupentixol) often have a strong D2 affinity; D4 data are heterogeneous and often come from older assays — therefore uncertain.
Recommendation for use
- Use these AI ranges for pharmacological classification (e.g., when D4 modulation is relevant to hypotheses).
- For precise comparisons or modeling, I recommend checking the original PDSP/primary data (note assay details) and considering metadata (radioligand, temperature, membrane source) NIMH PDSP - UNC.
Sources: NIMH PDSP - UNC APExBIO fishersci.de Psych Scene Hub benchchem.com MDPI.
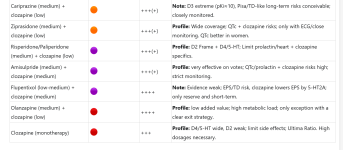
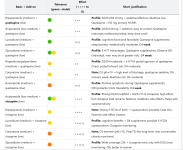
Add-on combinations by tolerability (primary), effect (secondary)
| Basic + Add-on | Tolerance (green→violet) | Effect (++++ to 0) | Short justification |
|---|---|---|---|
| Brexpiprazole (medium) + quetiapine (low) | +++ | Profile: D2/D3/D4 strong + sedative/affective; Akathisia low; Quetiapine <150 mg primary H1/M1. | |
| Aripiprazole (low-medium) + quetiapine (low) | +++ | Profile: D2/D4 strong + sedation; easy to control; Quetiapine intercepts restlessness/sleep, keep dose small. | |
| Lurasidone (medium) + quetiapine (low) | +(+) | Profile: cognitive/functional favorable; Quetiapine supplements sleep/anxiety; metabolically moderate. D4 weak. | |
| Ziprasidone (medium) + quetiapine (low) | +(+) | Profile: 5-HT7 Advantages; Quetiapine supplements; Observe QTc (individual, men may be at greater risk). D4 weak. | |
| Risperidone/paliperidone (medium) + quetiapine (low) | ++ | Profile: D2/D4 moderate + 5-HT1A partial agonism of quetiapine; Check prolactin/heart risk. D4 moderate. | |
| Cariprazine (medium) + quetiapine (low) | ++ | Note: D3 pKi≈10 → high level of blockage; quetiapine sedative, D4 remains weak; Akathisia risk. D4 moderate | |
| Amisulpride (medium) + quetiapine (low) | +(+) | Profile: Positive symptoms strong; Quetiapine supplements; QTc/prolactin limits tolerability. D4 weak. | |
| Aripiprazole (low-medium) + clozapine (low) | ++++ | Profile: Strong D2/D4 scaffold + D4/5-HT of clozapine; high effect, but clozapine load remains. Reduces metabolic side effects. Helps with supersensitivity. | |
| Brexpiprazole (medium) + clozapine (low) | ++++ | Note: Strong 5-HT2A of both → superposition possible; Data thin; Examine side effect clusters. | |
| Lurasidone (medium) + clozapine (low) | +++(+) | Profile: cognitive benefits + D4 supplement; possible 5-HT2A superposition; Clozapine monitoring. | |
| Cariprazine (medium) + clozapine (low) | +++(+) | Note: D3 extreme (pKi≈10), Pisa/TD-like long-term risks conceivable; closely monitored. | |
| Ziprasidone (medium) + clozapine (low) | +++(+) | Profile: Wide coverage; QTc + clozapine risks; only with ECG/close monitoring. QTc better in women. | |
| Risperidone/Paliperidone (medium) + clozapine (low) | +++(+) | Profile: D2 Frame + D4/5-HT; Limit prolactin/heart + clozapine specifics. | |
| Amisulpride (medium) + clozapine (medium) | +++(+) | Profile: very effective on votes; QTc/prolactin + clozapine risks high; strict monitoring. | |
| Flupentixol (low-medium) + clozapine (medium) | ++++ | Note: Evidence weak; EPS/TD risk, clozapine lowers EPS by 5-HT2A; only reserve and short-term. | |
| Olanzapine (medium) + clozapine (low) | ++++ | Profile: low added value; high metabolic load; only exception with a clear exit strategy. | |
| Clozapine (monotherapy) | +++ | Profile: D4/5-HT wide, D2 weak; limit side effects; Ultima Ratio. High dosages necessary. |
Prioritized recommendation track
- First line (tolerated + effective):
- Brexpiprazole + Quetiapine; Aripiprazole + Quetiapine.
- Reason: High D2/D3/D4 coverage by partial agonists, quetiapine low dose for sleep/anxiety; low side effect profile.
- Compatible alternatives:
- Lurasidone + Quetiapine; Ziprasidone + Quetiapine.
- Reason: Cognitive/functional benefits; Monitor QTc individually (especially with Ziprasidon).
- Reserve with clozapine (only when needed, low dose):
- Aripiprazole + clozapine (highly effective, orange), lurasidone/ziprasidone + clozapine (orange→red according to QTc), risperidone/paliperidone + clozapine (orange).
- Note: Always strict blood count/metabolic/ECG monitoring.
- Devaluations for reasons of tolerance:
- Cariprazine + quetiapine (orange) and cariprazine + clozapine (red) due to extremely high D3 blockade (pKi≈10) with potential long-term risks (akathisia/Pisa).
- Amisulpride + clozapine (red/purple) and flupentixol + clozapine (violet) only for a short time and monitored.
Practical dose and monitoring instructions
- Quetiapine as an add-on:
- Goal: below 150 mg in the evening.
- Use & Benefits: sleep, anxiety, affective stabilization; no D4 effect necessary, as the basic preparation is contributing.
- Partial agonists:
- Aripiprazole: 5–10 mg as a start; Observe the akathisia threshold.
- Brexpiprazole: can be dosed slightly higher than aripiprazole, lower in akathisia; 5-HT coverage stronger.
- Clozapine:
- Low dose as an add-on; Strict blood count (ANC), metabolic monitoring, myocarditis vigilance, salivation management.
- QTc/ECG:
- Baseline + follow-up for ziprasidone, amisulpride, olanzapine, clozapine combinations; examine gender-specific differences individually.
- Polypharmacy:
- >2 Avoid APs; only in the short term in the acute phase with a clear reduction strategy.
Quick Conclusion
- Quetiapine combinations with partial agonists (brexpiprazole, aripiprazole) are the most tolerable and at the same time highly effective first choice for therapy resistance.
- Clozapine remains an add-on of the ultima ratio, low-dose and strictly monitored when quetiapine strategies are inadequate.
- Cariprazine is devalued for tolerability reasons (extreme D3 blockade), especially in combination with clozapine.
- Amisulpride/clozapine and flupentixol/clozapine are effective, but only as a short-term reserve with high monitoring effort.
Monitoring and Security Aspects (Brief)
- Clozapine: Blood count (agranulocytosis), TDM recommended, metabolic parameters, sedation.
- Ziprasidon: ECG/QTc before and during therapy, electrolyte status.
- Olanzapine/clozapine combinations: close metabolic monitoring.
- Aripiprazole / Cariprazine: pay attention to akathisia/activation; Cariprazine: long half-life metabolite.
- General: dose as low as possible; check interactions; Observe kidney/liver function.
Note on the evidence: Ratings in the table are based on your specification combined with typical clinical experiences; The strength of evidence varies greatly between combinations (some are well studied, others only in case series). Concrete therapy decisions should always be made in an interdisciplinary and patient-specific manner.
Anhänge
Zuletzt bearbeitet: